Polymer Chemistry for Footwear Adhesive Guide
Introduction
In the world of footwear manufacturing, adhesives play a critical role in bonding materials like leather, rubber, and synthetics to create durable, comfortable shoes. But what drives their effectiveness? Polymer chemistry holds the answer. Polymers form the building blocks of many modern adhesives, delivering strength, flexibility, and resistance to wear. This blog post marks the first in our series on adhesives for footwear. We will explore the fundamentals of polymer chemistry, starting with monomers and polymers, their classifications, and various types commonly used. Stay tuned, for our next post will dive into different types of adhesives themselves.
Polymer Chemistry for Footwear Adhesive: Monomers and Polymers
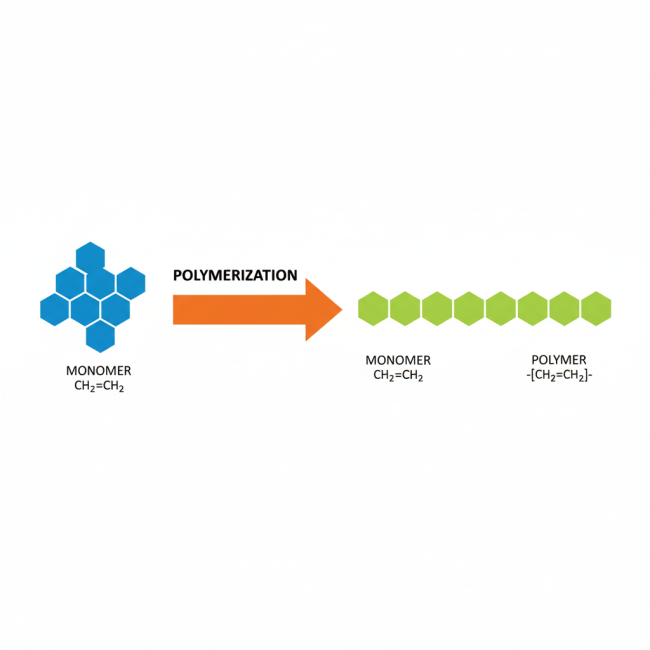
Polymer chemistry explores the study of large molecules formed by repeating smaller units. At its core, we find monomers and polymers.
- Monomer: A monomer acts as a small molecule that connects with other similar molecules to form a larger chain. Picture it as a single Lego brick—simple alone but capable of building complex structures when joined.
- Polymer: When monomers link together through chemical reactions, they create a polymer, a long chain or network of these repeating units. Polymers can be natural (like DNA or proteins) or synthetic (like plastics). In footwear adhesives, polymers provide the sticky, binding properties that hold components together under stress.
Chemists call the process of linking monomers polymerization, and it occurs in various ways, shaping different polymer structures and properties. For example, this process underpins the adhesives that secure shoe soles.
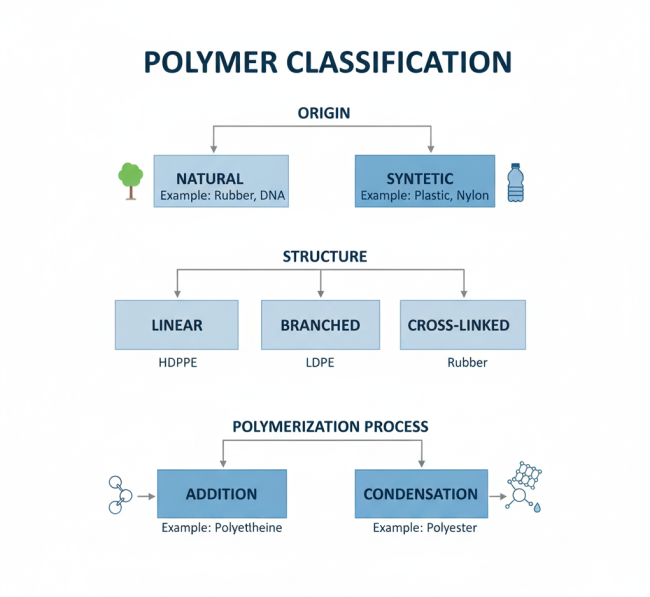
Classification of Polymers in Footwear Adhesive Chemistry
Polymers fall into categories based on several criteria, including their origin, structure, polymerization process, and molecular forces. Here’s a clear overview:
| Classification Basis | Categories | Examples |
| Origin | Natural, Synthetic | Natural: Rubber, Cellulose; Synthetic: Polyethylene, PVC |
| Structure | Linear, Branched, Cross-linked | Linear: Polyethylene; Cross-linked: Vulcanized rubber |
| Polymerization Process | Addition, Condensation | Addition: Polystyrene; Condensation: Polyamides |
| Molecular Forces | Elastomers, Fibers, Thermoplastics, Thermosets | Elastomers: Natural rubber; Thermoplastics: PVC |
This classification helps us select the right polymer for specific applications, such as flexible adhesives for shoe soles or rigid ones for structural bonding in footwear. In addition, understanding these categories guides manufacturers in choosing durable materials.
Addition Polymers in Footwear Adhesive Chemistry
One major type of polymer is the addition polymer. These emerge from the addition reaction of unsaturated monomers—typically those with double or triple bonds—without losing any small molecules. The process breaks the double bond and connects monomers in a chain.
- Scientists initiate the reaction with free radicals, anions, or cations.
- Properties: Often thermoplastic, meaning they melt and reshape easily.
In footwear, addition polymers like PVC offer water resistance and durability in adhesive formulations. Furthermore, their versatility makes them valuable in shoe production.ons.
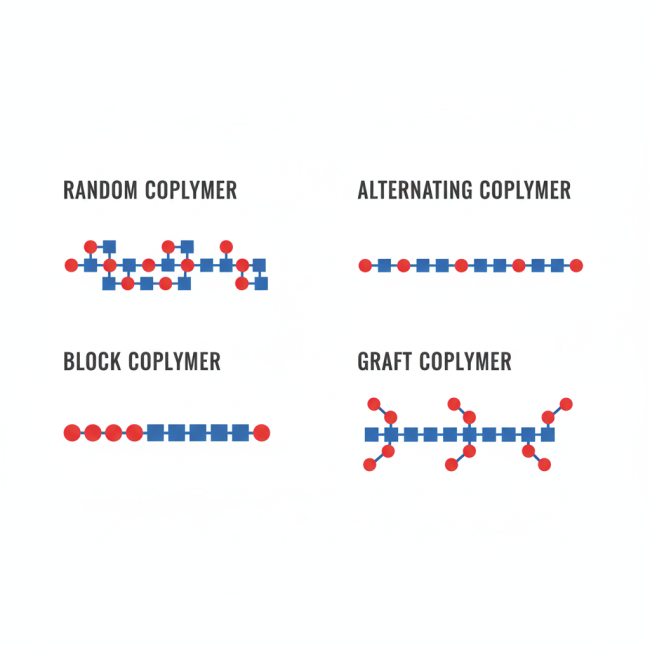
Copolymers and Their Role in Footwear Adhesive
While homopolymers arise from a single type of monomer, copolymers emerge as polymers derived from two or more different monomers. This combination allows tailored properties, such as improved adhesion or flexibility, which prove vital for footwear adhesives that bond diverse materials. In contrast, copolymers stand out for their adaptability.
Copolymers divide into classes based on the arrangement of monomer units:
- Random Copolymer: Monomers distribute randomly along the chain (e.g., styrene-butadiene rubber for flexible adhesives).
- Alternating Copolymer: Monomers alternate in a regular pattern.
- Block Copolymer: Large blocks of one monomer follow blocks of another (e.g., polyurethane block copolymers for elastic bonds).
- Graft Copolymer: One polymer chain attaches to the backbone of another.
These variations enable copolymers to show unique traits, making them ideal for specialized footwear applications like shock absorption or waterproofing. For instance, block copolymers enhance elasticity in athletic shoes.
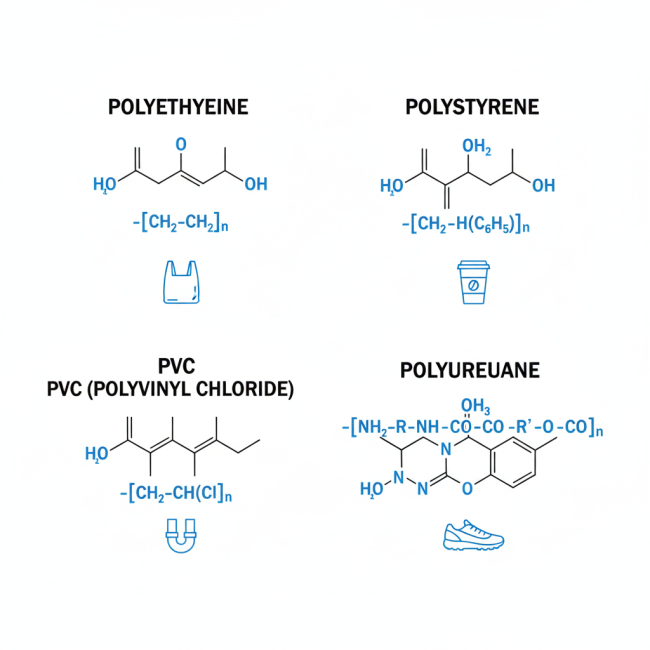
Different Types of Polymers in Footwear Adhesive Chemistry
Let’s cover some specific polymers mentioned, highlighting their structures, traits, and relevance to adhesives in footwear

- Polyethylene (PE): A simple addition polymer from ethylene monomers. It offers flexibility and water resistance, and manufacturers use it in adhesive films for shoe linings.
- Polystyrene (PS): Made from styrene, it provides rigidity and often appears in foamed adhesives for cushioning in footwear.
- Polyvinyl Chloride (PVC): An addition polymer from vinyl chloride, known for durability, and it supports solvent-based adhesives for bonding PVC soles.
- Bisphenol (e.g., Bisphenol A in Epoxy Resins): This forms cross-linked polymers, and industries apply it in strong, heat-resistant adhesives for high-performance shoes.
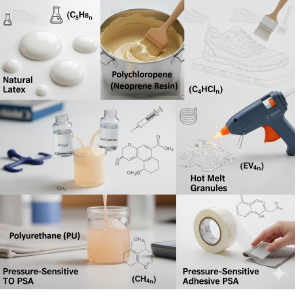
- Polyurethane (PU): A versatile copolymer from diisocyanates and polyols, it delivers flexible, elastic adhesives in athletic footwear.
- Phenol Formaldehyde: A thermoset resin from phenol and formaldehyde, it supplies heat-resistant bonds in industrial shoe assembly.
- Polyamides (e.g., Nylons): Condensation polymers from amines and acids, Nylons like Nylon 6,6 bring strength and support hot-melt adhesives for seams.
- Polyesters: Formed from diols and dicarboxylic acids, they provide good adhesion to fabrics and leathers in shoe uppers.
- Cellulose: A natural polymer from glucose units, its derivatives like cellulose acetate appear in eco-friendly adhesives.
- Natural Rubber: An elastomer from isoprene, it produces latex-based adhesives for bonding rubber soles, offering flexibility and grip.
These polymers form the basis of many adhesive formulations, and manufacturers choose them based on the footwear’s needs for strength, flexibility, and environmental resistance. Moreover, their diverse properties meet various design requirements.
Conclusion
Understanding polymer chemistry proves essential for grasping how adhesives function in footwear production. From basic monomers to complex copolymers, these materials enable the creation of bonds that withstand daily wear and tear. In our next blog, we will build on this foundation by exploring different types of adhesives specifically tailored for footwear applications. If you have questions or want to learn more, drop a comment below!
For more insights into manufacturing, subscribe to our blog.